Updates In Immunotherapy And Mrna Vaccines In Lung Cancer
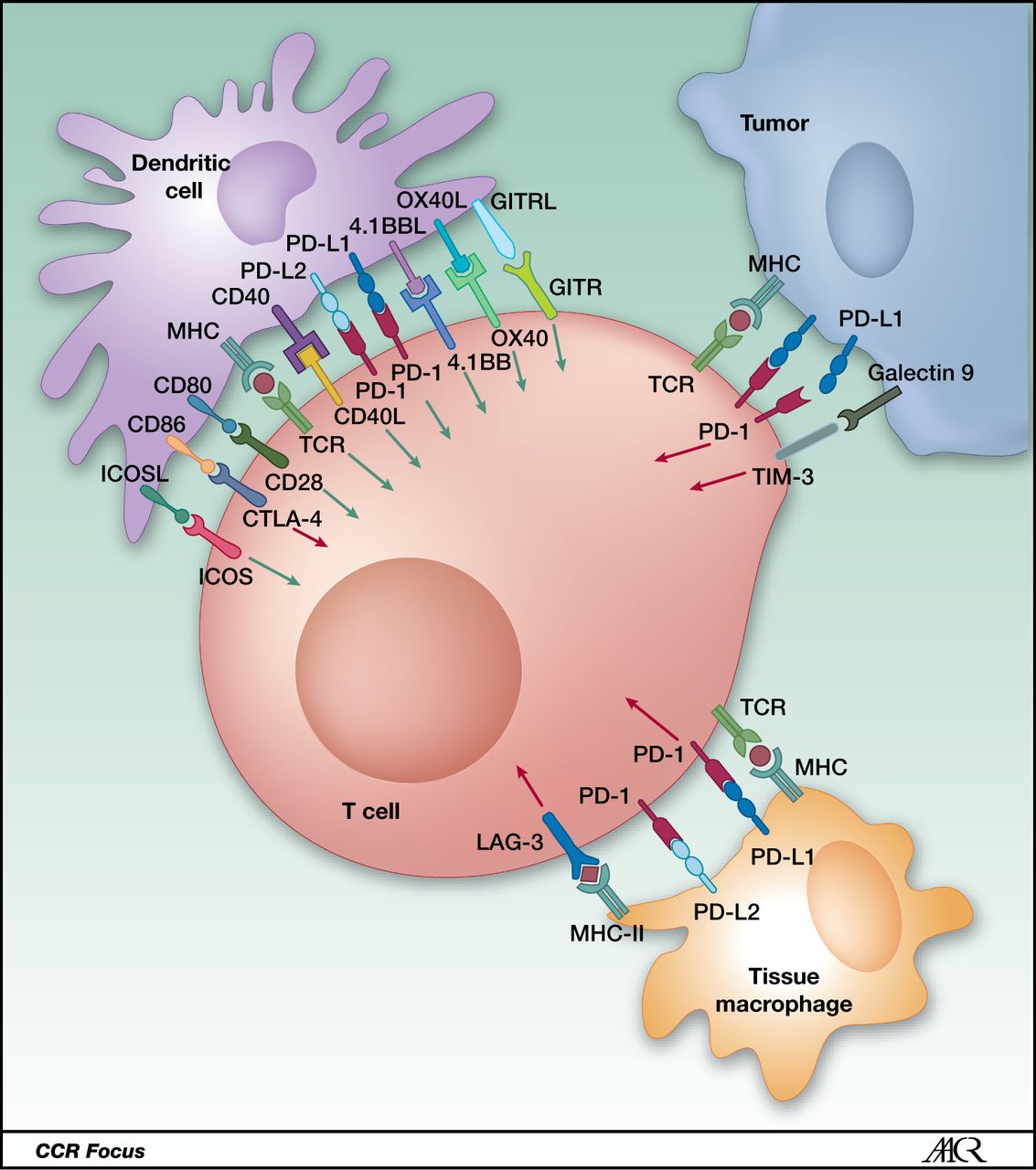
Cancer Immunotherapy Projections вђ Immune Checkpoint Inhibitors Lead Immune checkpoint inhibitor therapy, a type of immunotherapy, is often used to treat advanced non small cell lung cancer. in a phase 1b study led by jhanelle gray, md, chair of the department of thoracic oncology at moffitt cancer center, researchers evaluated the use of a messenger rna based cancer vaccine to improve upon immune checkpoint. Years of research exploring mrna vaccines for cancer treatment in preclinical and clinical trials have set the stage for the rapid development of mrna vaccines during the covid 19 pandemic. therapeutic cancer vaccines based on mrna are well tolerated, and the inherent advantage in ease of production, which rivals the best available conventional vaccine manufacture methods, renders mrna.
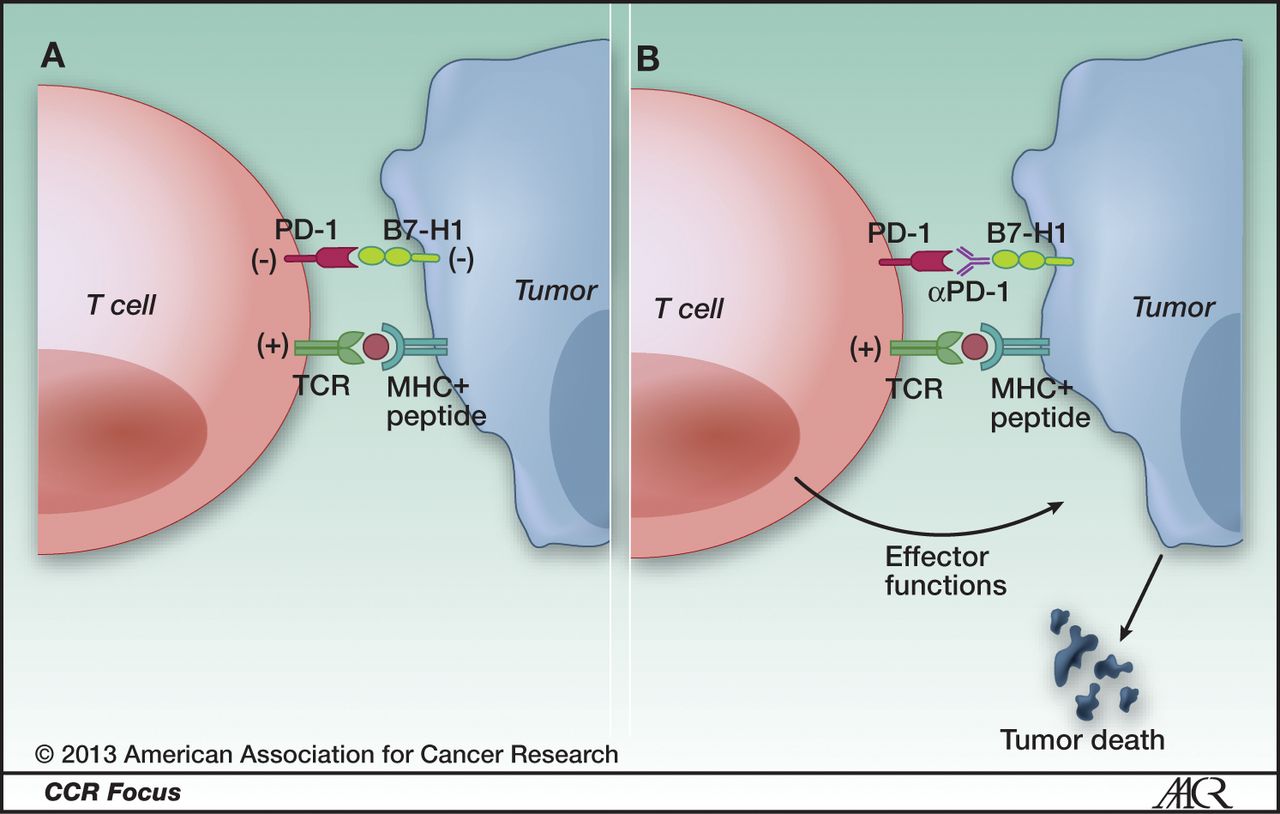
Cancer Immunotherapy Projections вђ Immune Checkpoint Inhibitors Lead Mrna vaccines present a tremendous opportunity to fulfil the promise of cancer immunotherapy first heralded as early as the late 1800s when william coley demonstrated that isolated bacterial. The phrase messenger rna and its acronym, mrna, have become familiar to the public during the covid 19 pandemic. the mrna vaccines for covid 19 work by instructing cells in the body how to make a protein that triggers an immune response against the virus. mrna technology has also been of interest to cancer researchers and physicians. Abstract. after several decades, therapeutic cancer vaccines now show signs of efficacy and potential to help patients resistant to other standard of care immunotherapies, but they have yet to. Owing to these exciting clinical findings, mrna 4157 is poised to become the world’s first mrna personalized tumor vaccine to undergo phase 3 clinical study. 98 notably, after the first patient.

Roche Signs 310mm Early Stage Deal With For Mrna Vaccines Cancer Biology Abstract. after several decades, therapeutic cancer vaccines now show signs of efficacy and potential to help patients resistant to other standard of care immunotherapies, but they have yet to. Owing to these exciting clinical findings, mrna 4157 is poised to become the world’s first mrna personalized tumor vaccine to undergo phase 3 clinical study. 98 notably, after the first patient. The bnt116 vaccine, which has been developed by the german biotechnology company biontech (mainz, germany) as an immunotherapy treatment for non small cell lung cancer (nsclc), uses mrna to provide information about common tumour markers to the patient's immune system, so that it is able to identify and mount an immune response to the cancer cells and prevent their return. Purpose to examine covid 19 mrna vaccine–induced binding and neutralizing antibody responses in patients with non–small cell lung cancer (nsclc) to sars cov 2 614d (wild type [wt]) strain and variants of concern after the primary 2 dose and booster vaccination. methods eighty two patients with nsclc and 53 healthy volunteers who received sars cov 2 mrna vaccines were included in the study.

Rna Vaccines In Melanoma And Prostate Cancer Cancer Biology The bnt116 vaccine, which has been developed by the german biotechnology company biontech (mainz, germany) as an immunotherapy treatment for non small cell lung cancer (nsclc), uses mrna to provide information about common tumour markers to the patient's immune system, so that it is able to identify and mount an immune response to the cancer cells and prevent their return. Purpose to examine covid 19 mrna vaccine–induced binding and neutralizing antibody responses in patients with non–small cell lung cancer (nsclc) to sars cov 2 614d (wild type [wt]) strain and variants of concern after the primary 2 dose and booster vaccination. methods eighty two patients with nsclc and 53 healthy volunteers who received sars cov 2 mrna vaccines were included in the study.

Towards An Effective Mrna Vaccine Against 2019 Ncov

Comments are closed.