The Properties Of Compressed Liquids Are Commonly Chegg
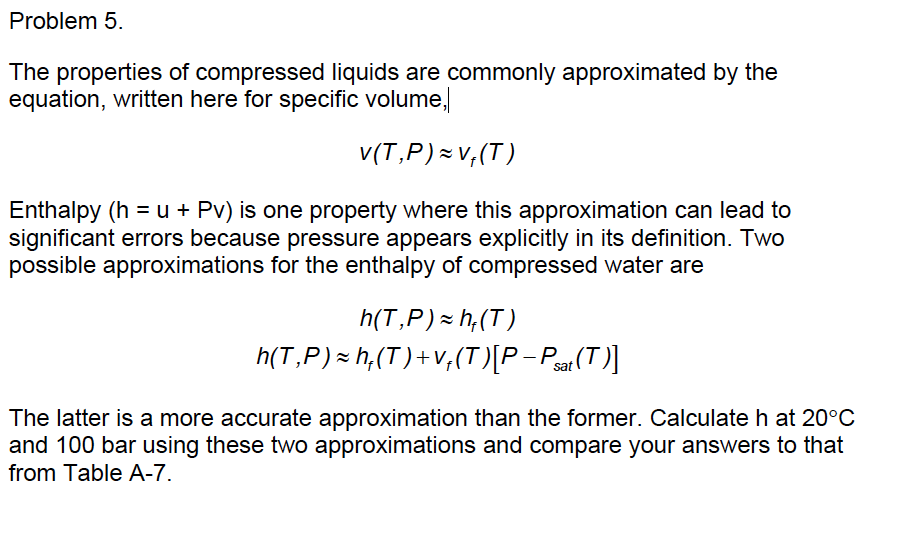
The Properties Of Compressed Liquids Are Commonly Chegg Question: the properties of compressed liquids are commonly approximated by the equation, written here for specific volume, v (t ,p ) = vf (t ) enthalpy (h = u pv) is one property where this approximation can lead to significant errors because pressure appears explicitly in its definition. two possible approximations for the enthalpy of. Answer to the properties of compressed liquids are commonly. the properties of compressed liquids are commonly approximated by the equation, written here for specific volume, v(t ,p ) =v f (t ) enthalpy (h = u pv) is one property where this approximation can lead to significant errors because pressure appears explicitly in its definition.

Solved For The Compressed Liquid What Property Ies Is Chegg Compressed liquid propertiesincompressible fluidscompressed liquid tablesvf for vhf for hsf for s0:00 changes in properties for compressed liquid0:56 incompr. Critical point properties table a–2 ideal gas specific heats of various common gases table a–3 properties of common liquids, solids, and foods table a–4 saturated water—temperature table table a–5 saturated water—pressure table table a–6 superheated water table a–7 compressed liquid water table a–8 saturated ice–water vapor. In this chapter we consider the property values and relationships of a pure substance (such as water) which can exist in three phases – solid, liquid and gas. we will not consider the solid phase in this course.in order to introduce the rather complex phase change interactions that occur in pure substances we consider an experiment in which. The viscosity of a liquid is a measure of its resistance to flow. water, gasoline, and other liquids that flow freely have a low viscosity. honey, syrup, motor oil, and other liquids that do not flow freely, like those shown in figure 10.2.1 10.2. 1, have higher viscosities. we can measure viscosity by measuring the rate at which a metal ball.

Solved This Is Thermodynamics Chegg In this chapter we consider the property values and relationships of a pure substance (such as water) which can exist in three phases – solid, liquid and gas. we will not consider the solid phase in this course.in order to introduce the rather complex phase change interactions that occur in pure substances we consider an experiment in which. The viscosity of a liquid is a measure of its resistance to flow. water, gasoline, and other liquids that flow freely have a low viscosity. honey, syrup, motor oil, and other liquids that do not flow freely, like those shown in figure 10.2.1 10.2. 1, have higher viscosities. we can measure viscosity by measuring the rate at which a metal ball. Lets look at 11.6.9. if t 1 <t 2, then p 1 <p 2 , the 1 (t 1 t 2) term is negative. p 1 p 2 ,is a fraction and ln of a fraction is a negative number. this means the enthalpy of vaporization is a positive number as dividing a negative number into a positive number gives a negative number, and it makes sense. The liquid vapour phase change can be illustrated in the and diagrams, as shown in figures 2.3.4 and 2.3.5. in these diagrams, we can clearly see the three regions: compressed liquid region, saturated liquid vapour region, and superheated vapour region. the curve that separates the compressed liquid region and saturated liquid vapour region is.

Solved Which Properties Of A Compressed Liquid At A Given Chegg Lets look at 11.6.9. if t 1 <t 2, then p 1 <p 2 , the 1 (t 1 t 2) term is negative. p 1 p 2 ,is a fraction and ln of a fraction is a negative number. this means the enthalpy of vaporization is a positive number as dividing a negative number into a positive number gives a negative number, and it makes sense. The liquid vapour phase change can be illustrated in the and diagrams, as shown in figures 2.3.4 and 2.3.5. in these diagrams, we can clearly see the three regions: compressed liquid region, saturated liquid vapour region, and superheated vapour region. the curve that separates the compressed liquid region and saturated liquid vapour region is.

Solved Properties Of Compressed Liquid Watertable A 3tables Chegg

Comments are closed.