S1 3 1 The Hydrogen Emission Spectrum

S1 3 1 The Hydrogen Emission Spectrum Youtube An explanation of continuous & line spectra and the hydrogen emission spectrum.0:00 white light produces a continuous spectrum0:49 excited hydrogen gas produ. Figure 1.3.3: the emission spectra of elements compared with hydrogen. these images show (a) hydrogen gas, which is atomized to hydrogen atoms in the discharge tube; (b) helium; and (c) mercury. the strongest lines in the hydrogen spectrum are in the far uv lyman series starting at 124 nm and below.

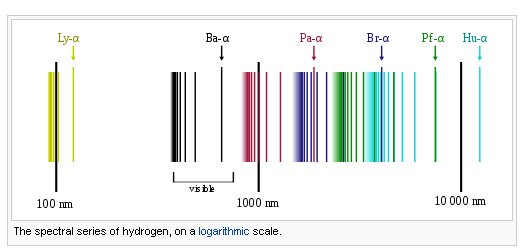
S1 3 2 The Hydrogen Emission Spectrum Youtube Hydrogen emission spectrum. the hydrogen emission spectrum is particularly important. it consists of several series of lines, each corresponding to electrons transitioning to a particular energy level: lyman series (ultraviolet): transitions to n = 1; balmer series (visible): transitions to n = 2; paschen series (infrared): transitions to n = 3. Δe = = = hν (6.626 ×10−34)(3.28 ×1015) 2.173 ×10−18 j (4) (5) (6) this is the ionization energy for a single atom. to find the normally quoted ionization energy, this value is multiplied by the number of atoms in a mole of hydrogen atoms (the avogadro constant) and then dividing by 1000 to convert joules to kilojoules. Substituting the appropriate values of r h, n 1, and n 2 into the equation shown above gives the following result. solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental value of 486.1 nm for the blue line in the visible spectrum of the hydrogen atom. Hydrogen molecules are first broken up into hydrogen atoms (hence the atomic hydrogen emission spectrum) and electrons are then promoted into higher energy levels. suppose a particular electron was excited into the third energy level. this would tend to lose energy again by falling back down to a lower level.
Emission Spectrum Of The Hydrogen Atom Introduction To Chemistry Substituting the appropriate values of r h, n 1, and n 2 into the equation shown above gives the following result. solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental value of 486.1 nm for the blue line in the visible spectrum of the hydrogen atom. Hydrogen molecules are first broken up into hydrogen atoms (hence the atomic hydrogen emission spectrum) and electrons are then promoted into higher energy levels. suppose a particular electron was excited into the third energy level. this would tend to lose energy again by falling back down to a lower level. The general formula for the hydrogen emission spectrum is given by: where, n 1 = 1,2,3,4 … n 2 = n 1 1. ν= wave number of electromagnetic radiation. the value 109,677 cm 1 is known as rydberg constant for hydrogen. to learn more about hydrogen emission spectrum download byju’s – the learning app. read more: emission spectrum and atomic. Structure 1.3.2 the line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies. describe the emission spectrum of the hydrogen atom, including the relationships between the lines and energy transitions to the first, second and third energy levels.

Solution Chemistry Notes Hydrogen Emission Spectrum Studypool The general formula for the hydrogen emission spectrum is given by: where, n 1 = 1,2,3,4 … n 2 = n 1 1. ν= wave number of electromagnetic radiation. the value 109,677 cm 1 is known as rydberg constant for hydrogen. to learn more about hydrogen emission spectrum download byju’s – the learning app. read more: emission spectrum and atomic. Structure 1.3.2 the line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies. describe the emission spectrum of the hydrogen atom, including the relationships between the lines and energy transitions to the first, second and third energy levels.

Comments are closed.