Periodic Table Of Elements Alkali Metals
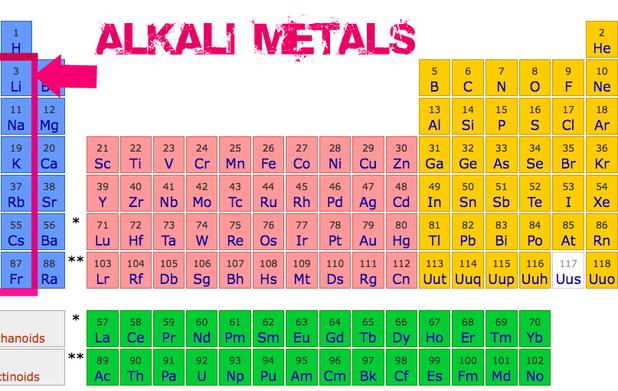
The General Properties Of The Alkali Metals In The Modern Periodic Alkali metal, any of the six elements of group 1 (ia) of the periodic table—lithium, sodium, potassium, rubidium, cesium, and francium. the alkali metals are so called because reaction with water forms alkalies (i.e., strong bases capable of neutralizing acids). Alkali metals are the six different chemical elements found in the first column of the periodic table: lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). the alkali metals group is part of the s block of elements in the periodic table , that along with hydrogen, helium, calcium and others, have their outermost electron in an s orbital.

Basic Types Of Metals On The Periodic Table The alkali metals are among the most electropositive elements on the periodic table and thus tend to bond ionically to the most electronegative elements on the periodic table, the halogens (fluorine, chlorine, bromine, iodine, and astatine), forming salts known as the alkali metal halides. the reaction is very vigorous and can sometimes result in explosions. The alkali metals are the elements located in group ia of the periodic table (the first column). the key characteristic these elements share in common is that they all have one electron in the outer electron shell. this lone electron is loosely bound, making this a set of reactive metallic elements. list of the alkali metals. The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. alkali metals have one electron in their outer shell, which is loosely bound. this gives them the largest atomic radii of the elements in their respective periods. their low ionization energies result in. The alkali metals, also known as the alkali metal family is a group of six elements characterized by common physical and chemical properties, a similar electron configuration, and shared periodic trends. all the alkali metals are found in nature, but being highly reactive, they do not occur freely in their pure form [1].
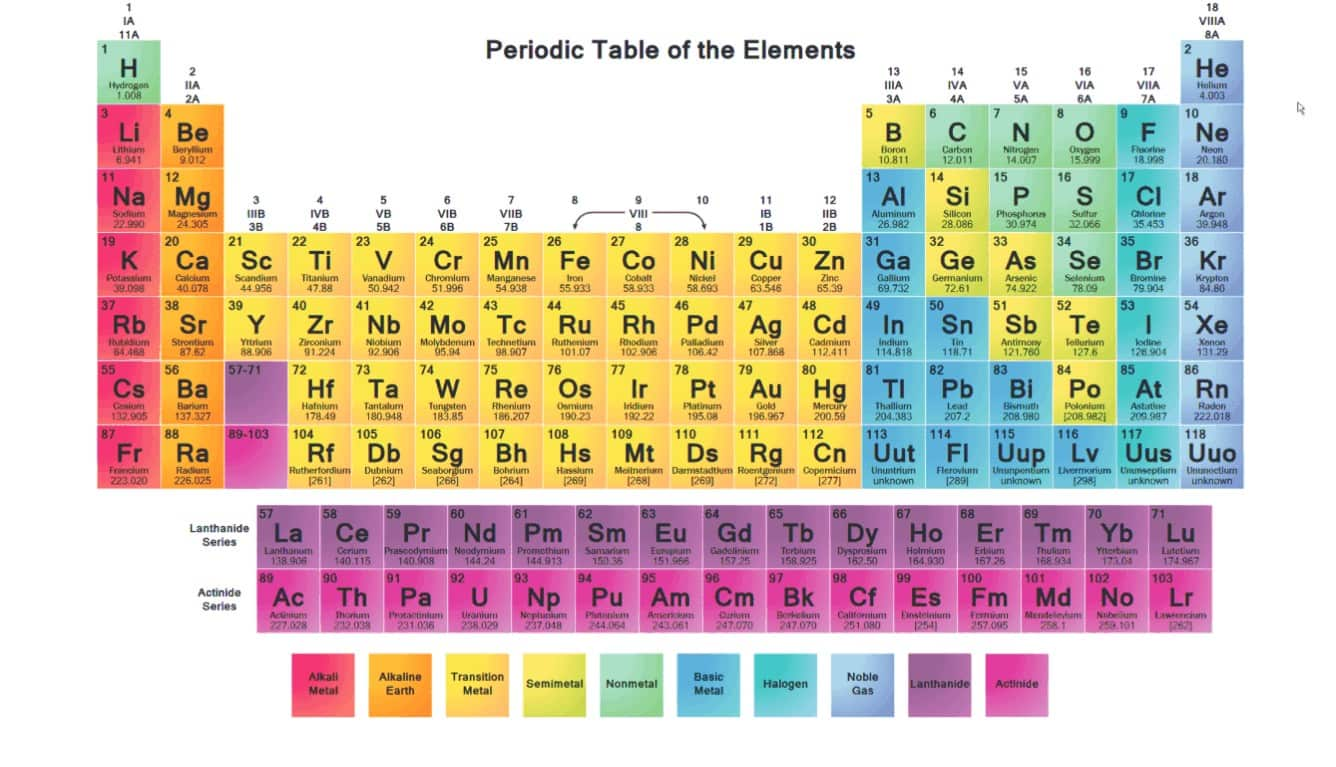
Alkali Metals Chemical Elements Properties Alkali Metals Periodicођ The alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. alkali metals have one electron in their outer shell, which is loosely bound. this gives them the largest atomic radii of the elements in their respective periods. their low ionization energies result in. The alkali metals, also known as the alkali metal family is a group of six elements characterized by common physical and chemical properties, a similar electron configuration, and shared periodic trends. all the alkali metals are found in nature, but being highly reactive, they do not occur freely in their pure form [1]. Further, as can be seen from the data in table 8.3.1 8.3. 1, both the melting and boiling points decrease down the alkali metal group. this decrease in melting and boiling points reflects a decrease in metallic bonding strength as the atomic size (and consequently average electron nucleus distance) increases down the group. table 8.3.1 8.3. The group 1 close group 1 the first vertical column of elements in the periodic table, starting with lithium and ending with francium. also called the alkali metals. elements close element a.

Comments are closed.