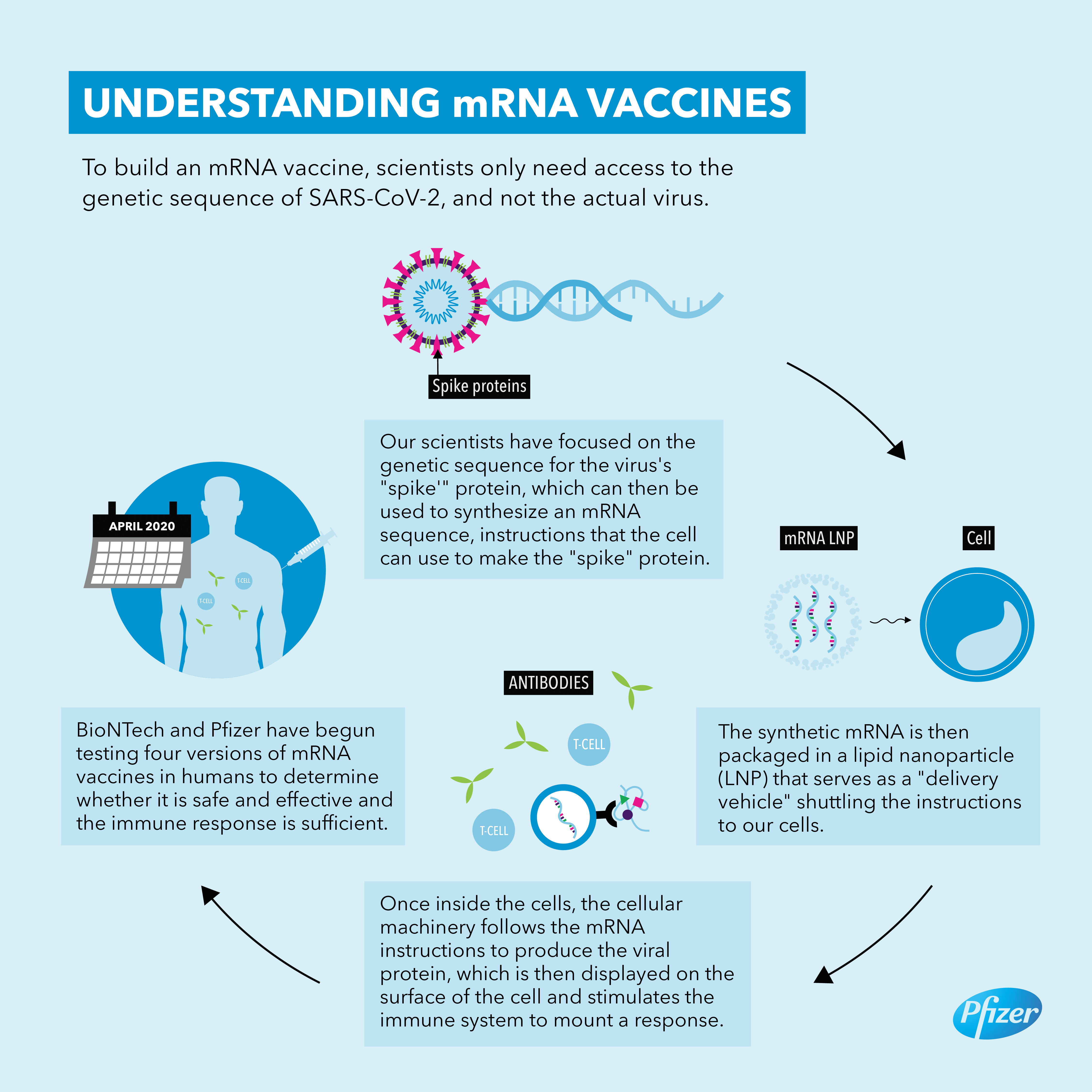
Mrna Vaccine Advances
Pfizer And Biontech Dose First Participants In The U S As Part Of The successful development of mrna vaccines is a result of years of research and groundwork. the mrna molecule was first described by brenner and colleagues in 1961, 60 and due to the highly. Cancer mrna vaccines: clinical advances and future opportunities. elias j. sayour, david boczkowski, duane a. mitchell &. smita k. nair. nature reviews clinical oncology 21, 489–500 (2024) cite.

Clinical Advances And Ongoing Trials Of Mrna Vaccines For Cancer Advances in mrna vaccine technology have facilitated their rapid design and production against emerging infectious diseases. orf sequences have been optimized to enhance mrna stability and. Technological advances have optimised mrna based vaccine stability, structure, and delivery methods, and multiple clinical trials investigating mrna vaccine therapy are now enrolling patients with various cancer diagnoses. although therapeutic mrna based cancer vaccines have not yet been approved for standard treatment, encouraging results from. On august 23, 2021, the pfizer biontech was the first covid 19 vaccine officially approved for commercialization by the fda, 105 being also the first ever approved on october 29, 2021 for use in children aged 5–11. 106 thus, the mrna vaccine technology has the most promising application prospects for covid 19. Various techniques have been employed in the development of covid 19 vaccines. among these, the covid 19 messenger rna (mrna) vaccine has been drawing increasing attention owing to its great application prospects and advantages, which include short development cycle, easy industrialization, simple production process, flexibility to respond to.

Comments are closed.