How To Write Molecular Formula Of Cupric Oxide Chemical Formula Of Cupric Oxide

How To Write Molecular Formula Of Cupric Oxide Chemical Formu In this video we'll write the correct formula for copper (ii) oxide (cuo).to write the formula for copper (ii) oxide we’ll use the periodic table and follow. Copper (ii) oxide or cupric oxide is an inorganic compound with the formula cuo. a black solid, it is one of the two stable oxides of copper, the other being cu 2 o or copper (i) oxide (cuprous oxide). as a mineral, it is known as tenorite, or so e times black copper. it is a product of copper mining and the precursor to many other copper.
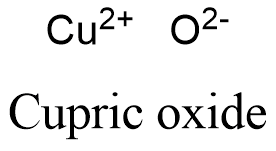
Cupric Oxide Formula The following steps should be taken while attempting to write a formula. the method is writing chemical formulae every compound is represented by a formula. the following steps should be taken. Chemical structure and properties. as the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). the ‘ii’ in its name signifies that the copper ion in this compound has a 2 oxidation state. this is a result of copper losing two of its electrons to oxygen, which is more electronegative. Copper (ii) oxide is made of two ions; the cupric ion, cu 2, and the oxygen ion, o 2. one cupric ion transfers its two electrons to the oxygen ion. cu 2 o 2 → cuo. one cupric ion is required. Teaching notes. students should have recorded the following masses: (mass 1) test tube bung. (mass 2) test tube bung copper oxide. (mass 3) test tube bung copper (product) this should allow them to calculate the mass of the mass of the copper oxide (mass 2) – (mass 1) and the mass of the copper (mass 3) – (mass 1).

Comments are closed.