Differences Between Cuprous Oxide Cu2o And Copper Cupric Oxide C

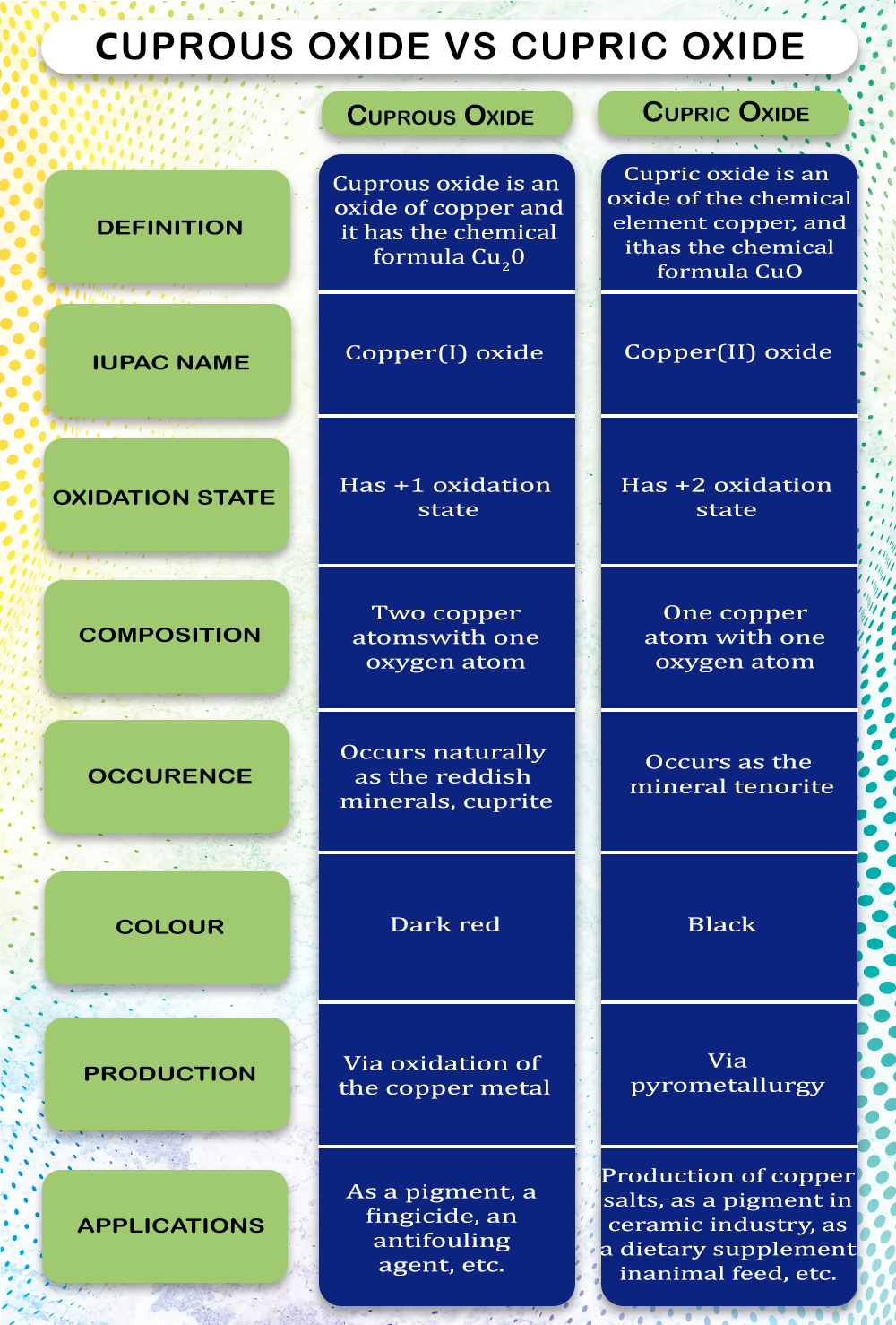
Differences Between Cuprous Oxide Cu2o And Copper Cupric о If only one atom of copper bonds to an oxygen molecule, it is called cupric oxide. if two copper atoms bond to an oxygen atom, it is cuprous oxide. cupric oxide is considered "fully oxidized," while cuprous oxide is still in an active state. the key to cuprous oxide, the aspect that makes it extremely effective as a biocide, is that active state. Key differences. cuprous oxide is characterized by its red color and is copper (i) oxide, where copper has a 1 oxidation state. in contrast, cupric oxide is black and is copper (ii) oxide, with copper in a 2 oxidation state. both are compounds of copper and oxygen but differ in their copper oxidation states. in cuprous oxide, each copper atom.

Differences Between Cuprous Oxide Cu2o And Copper Cupric о 24. transition metals and coordination compounds 3h 16m. copper corrodes to cuprous oxide, cu2o, or cupric oxide, cuo, depending on environmental conditions. Copper (ii) oxide or cupric oxide is an inorganic compound with the formula cuo. a black solid, it is one of the two stable oxides of copper, the other being cu 2 o or copper (i) oxide (cuprous oxide). as a mineral, it is known as tenorite, or so e times black copper. it is a product of copper mining and the precursor to many other copper. Conclusion. in conclusion, cupric and cuprous represent different oxidation states of copper, each with its own unique attributes and applications. cupric is a blue green solid, while cuprous is a reddish brown solid. cupric compounds are generally more stable and less reactive, finding applications in agriculture, pigments, and batteries. Infobox references. copper (i) oxide or cuprous oxide is the inorganic compound with the formula cu 2 o. it is one of the principal oxides of copper, the other being copper (ii) oxide or cupric oxide (cuo).the compound can appear either yellow or red, depending on the size of the particles. [ 2 ] cuprous oxide is found as the mineral cuprite.
Types Of юааcopperюаб юааoxideюаб And Itтащs Uses Conclusion. in conclusion, cupric and cuprous represent different oxidation states of copper, each with its own unique attributes and applications. cupric is a blue green solid, while cuprous is a reddish brown solid. cupric compounds are generally more stable and less reactive, finding applications in agriculture, pigments, and batteries. Infobox references. copper (i) oxide or cuprous oxide is the inorganic compound with the formula cu 2 o. it is one of the principal oxides of copper, the other being copper (ii) oxide or cupric oxide (cuo).the compound can appear either yellow or red, depending on the size of the particles. [ 2 ] cuprous oxide is found as the mineral cuprite. At medium and high temperatures 337–345 copper ultimately follows the parabolic law. 346 it has been shown using radioactive tracers 347,348 that the diffusion of copper ions in cuprous oxide is the rate determining step at 800–1000 °c, and there is considerable evidence in favor of the view that metal ions move outwards through the film by means of vacant sites in the oxide lattice. 133. Copper (i) oxide is also called as cuprous oxide, an inorganic compound with the chemical formula cu 2 o. it is covalent in nature. copper (i) oxide crystallizes in a cubic structure. it is easily reduced by hydrogen when heated. it undergoes disproportionation in acid solutions producing copper (ii) ions and copper.

Comments are closed.