Cu Forms Two Oxides Cuprous And Cupric Filo
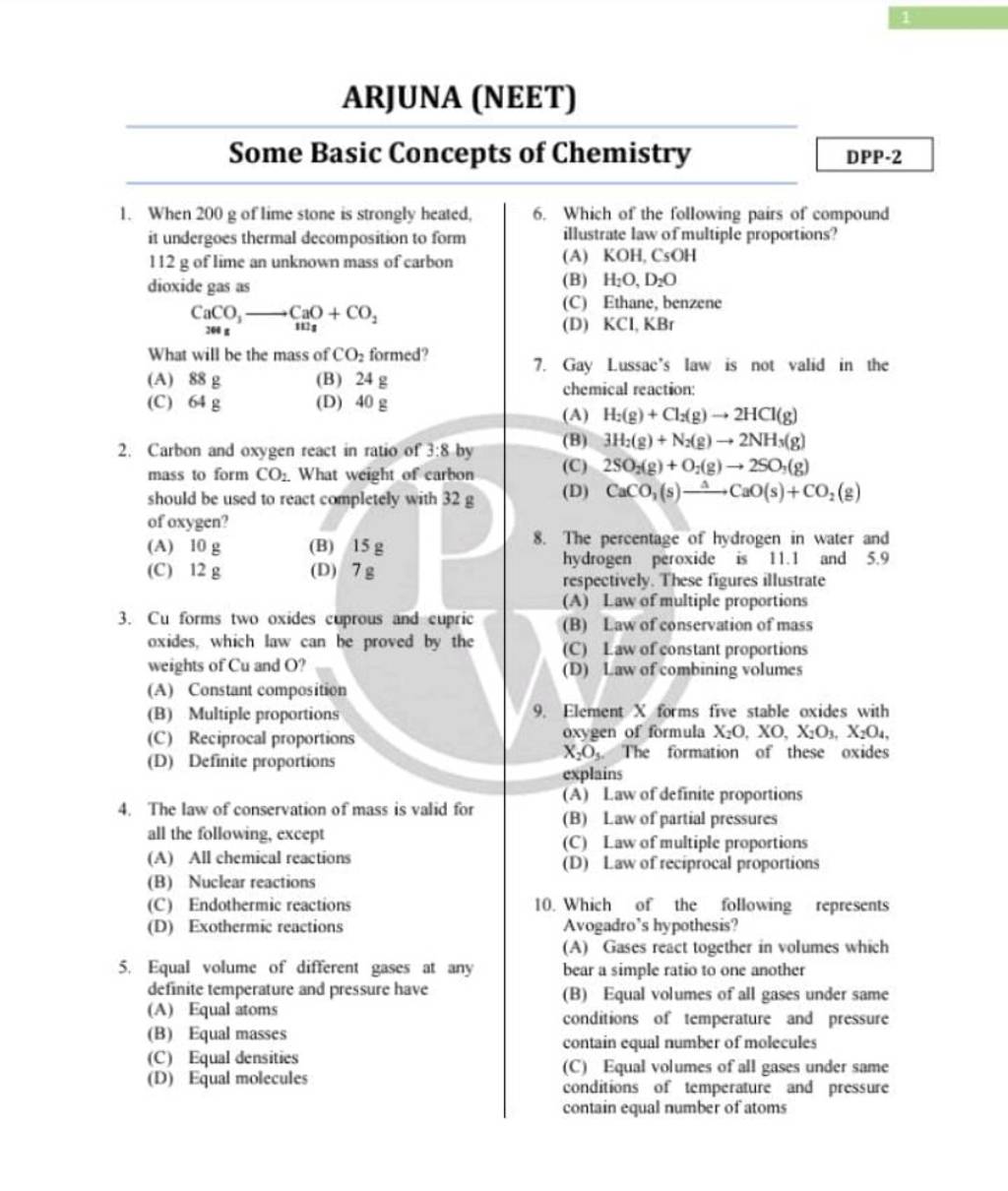
Cu Forms Two Oxides Cuprous And Cupric Filo Solution for cu forms two oxides cuprous and cupric oxides, which law can be proved by the weights of cu and o?a. constant composition b. multiple proporations c. reciprocal proportions. Solution for cu forms two oxides cuprous and cupric oxides, learn from their 1 to 1 discussion with filo tutors. 9 mins. uploaded on:.
Cu Forms Two Oxides Cuprous And Cupric O Xide Which Law Ca Solution for cu forms two oxides cuprous and cupric get 2 free instant explanations on filo with code filoapp. send. home. cbse. class 12 passed. chemistry. Q. phosphorus forms two oxides p 2o3 and p 2o5. which law can be proved by the weights of p and o? q. assertion :cuprous ion (cu ) is colourless whereas cupric ion (cu 2 ) produces blue colour in solution. reason: cuprous ion (cu ) has unpaired electrons while cupric ion (cu 2 ) does not. q. Conclusion. in conclusion, cupric and cuprous represent different oxidation states of copper, each with its own unique attributes and applications. cupric is a blue green solid, while cuprous is a reddish brown solid. cupric compounds are generally more stable and less reactive, finding applications in agriculture, pigments, and batteries. Cu(h2o)6]2 4cl − ⇌ [cucl4]2 − 6h2o. because the reaction is reversible, you get a mixture of colors due to both of the complex ions. the color of the tetrachlorocuprate (ii) ion may also be described as olive green or yellow. if you add water to the green solution, it returns to the blue color.

Cu Forms Two Oxides Cuprous And Cupric Oxides Which Law Ca Conclusion. in conclusion, cupric and cuprous represent different oxidation states of copper, each with its own unique attributes and applications. cupric is a blue green solid, while cuprous is a reddish brown solid. cupric compounds are generally more stable and less reactive, finding applications in agriculture, pigments, and batteries. Cu(h2o)6]2 4cl − ⇌ [cucl4]2 − 6h2o. because the reaction is reversible, you get a mixture of colors due to both of the complex ions. the color of the tetrachlorocuprate (ii) ion may also be described as olive green or yellow. if you add water to the green solution, it returns to the blue color. Copper (ii) compounds of commercial value include cupric oxide (cuo), cupric chloride (cucl 2), and cupric sulfate (cuso 4). cupric oxide is a black powder that occurs as the minerals tenorite and paramelaconite. large amounts are produced by roasting mixed copper oxide ores in a furnace at a temperature below 1,030 °c (1,900 °f). If only one atom of copper bonds to an oxygen molecule, it is called cupric oxide. if two copper atoms bond to an oxygen atom, it is cuprous oxide. cupric oxide is considered "fully oxidized," while cuprous oxide is still in an active state. the key to cuprous oxide, the aspect that makes it extremely effective as a biocide, is that active state.

Cu Forms Two Oxides Cuprous And Cupric Oxides Which Law Ca Copper (ii) compounds of commercial value include cupric oxide (cuo), cupric chloride (cucl 2), and cupric sulfate (cuso 4). cupric oxide is a black powder that occurs as the minerals tenorite and paramelaconite. large amounts are produced by roasting mixed copper oxide ores in a furnace at a temperature below 1,030 °c (1,900 °f). If only one atom of copper bonds to an oxygen molecule, it is called cupric oxide. if two copper atoms bond to an oxygen atom, it is cuprous oxide. cupric oxide is considered "fully oxidized," while cuprous oxide is still in an active state. the key to cuprous oxide, the aspect that makes it extremely effective as a biocide, is that active state.

Comments are closed.