Chemical Properties Of Metals With Reaction Examples Teachoo
Chemical Properties Of Metals With Reaction Examples Teachoo The reactions for these metals are given below. metal acid > metal sulphate hydrogen gas. similarly, other salts such as zinc, iron, aluminium and magnesium react with dil. h 2 so 4 to give their metal sulphates and hydrogen gas. metals like gold, copper, silver and platinum do not react with dil. h 2 so 4. Last updated at april 16, 2024 by teachoo. some metals react with bases to produce salt and hydrogen gas. note : metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. but all metals do not react with bases to produce hydrogen gas. some non metals react with bases but no hydrogen gas is produced.
---teachoo.png)
Chemical Properties Of Metals With Reaction Examples Teachoo Thereafter the physical properties, you are going to learn about the chemical properties of metals and non metals. chemical properties will include reaction of metals in air. reaction of metals in water. reaction of metals with other metals . lastly, you are going to learn about the reactivity of various metals and how they are different from. Metals exhibit a wide range of chemical properties that distinguish them from non metals. these properties are a result of their tendency to lose electrons and form positive ions. below is a detailed explanation of the chemical properties of metals along with examples, applications, and practice questions. reaction of metals with oxygen. The chemical properties of metals and nonmetals are described below. let us start with the chemical properties of metals. the density of metals is usually high. metals are malleable and ductile. metals form an alloy with other metals or non – metals. some metals react with air and corrode. for e.g. iron. Metals are the electropositive element that tends to donate electrons and form positive ions and become stable. for example: na → na e –. metals also generally have certain distinguishing physical properties that make them easy to identify and classify. some main physical properties of metals are; they have lustre and a metallic appearance.

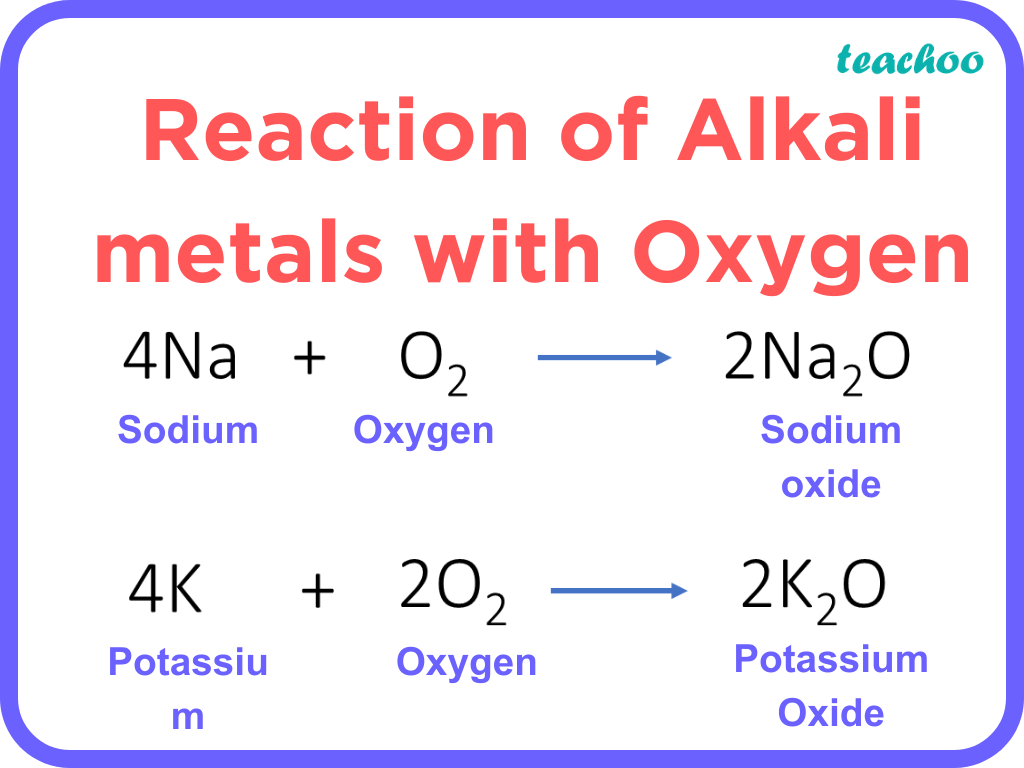
Chemical Properties Of Metals With Reaction Examples Teachoo The chemical properties of metals and nonmetals are described below. let us start with the chemical properties of metals. the density of metals is usually high. metals are malleable and ductile. metals form an alloy with other metals or non – metals. some metals react with air and corrode. for e.g. iron. Metals are the electropositive element that tends to donate electrons and form positive ions and become stable. for example: na → na e –. metals also generally have certain distinguishing physical properties that make them easy to identify and classify. some main physical properties of metals are; they have lustre and a metallic appearance. Usually, all the metals have a shiny appearance but these metals can also be polished to have a shiny appearance. chemical properties of metals. reaction with water: only highly reactive metals react with water and not all the metals. for example, sodium reacts vigorously with water and oxygen and gives a large amount of heat in the process. Chemical properties of metals: some of the chemical properties of metals are given below. reaction with oxygen – metals react with oxygen and form metallic oxides which are basic in nature. metal oxygen metal oxide . metal oxide water base . example –magnesium is metal and when it reacts with oxygen, it forms magnesium oxide.

Chemical Properties Of Metals With Reaction Examples Teachoo Usually, all the metals have a shiny appearance but these metals can also be polished to have a shiny appearance. chemical properties of metals. reaction with water: only highly reactive metals react with water and not all the metals. for example, sodium reacts vigorously with water and oxygen and gives a large amount of heat in the process. Chemical properties of metals: some of the chemical properties of metals are given below. reaction with oxygen – metals react with oxygen and form metallic oxides which are basic in nature. metal oxygen metal oxide . metal oxide water base . example –magnesium is metal and when it reacts with oxygen, it forms magnesium oxide.

Chemical Properties Of Metals With Reaction Examples Teachoo

Comments are closed.