Bohr Model Of The Hydrogen Atom Electron Transitions Atomic Energy Levels Lyman Balmer Series

Bohr Model Of The Hydrogen Atom Electron Transitions Atomicођ This chemistry video tutorial focuses on the bohr model of the hydrogen atom. it explains how to calculate the amount of electron transition energy that is. The index n that enumerates energy levels in bohr’s model is called the energy quantum number. we identify the energy of the electron inside the hydrogen atom with the energy of the hydrogen atom. note that the smallest value of energy is obtained for n = 1, n = 1, so the hydrogen atom cannot have energy smaller than that.
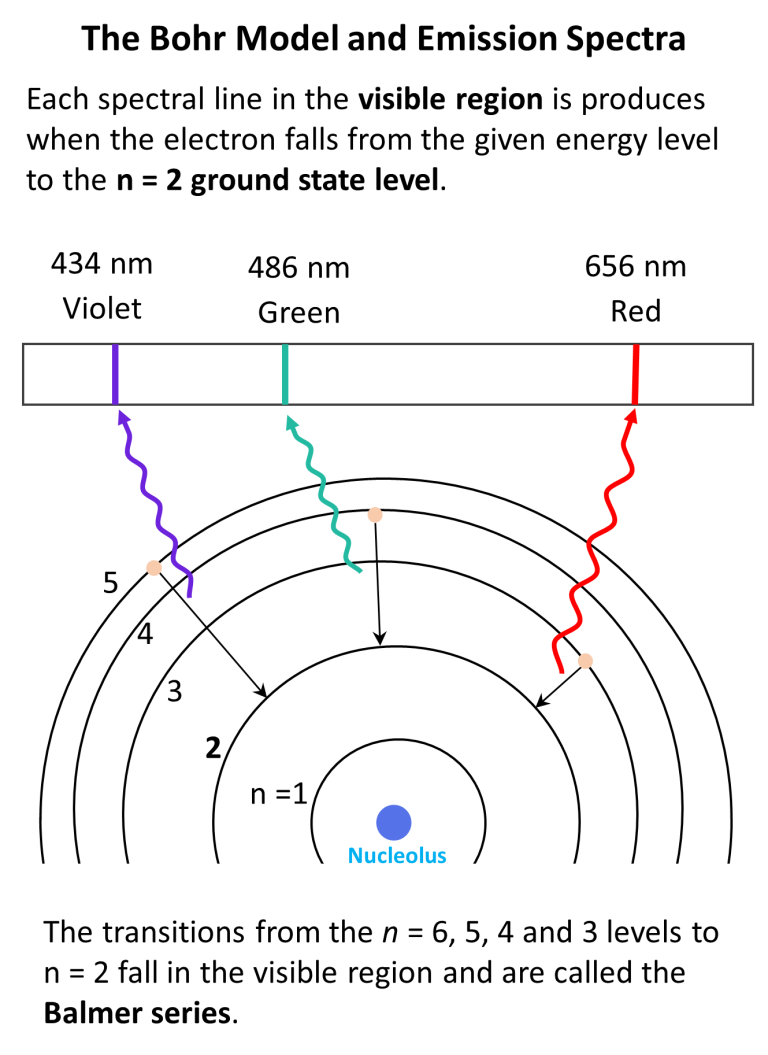
Bohr Model Of The Hydrogen Atom Chemistry Steps The electron’s speed is largest in the first bohr orbit, for n = 1, which is the orbit closest to the nucleus. the radius of the first bohr orbit is called the bohr radius of hydrogen, denoted as a0. its value is obtained by setting n = 1 in equation 6.5.6: a0 = 4πϵ0 ℏ2 mee2 = 5.29 × 10 − 11m = 0.529 Å. The bohr model was later replaced by quantum mechanics in which the electron occupies an atomic orbital rather than an orbit, but the allowed energy levels of the hydrogen atom remained the same as in the earlier theory. spectral emission occurs when an electron transitions, or jumps, from a higher energy state to a lower energy state. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. according to bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. each orbit has its specific energy level, which is expressed as a negative value. this is because the electrons on the orbit are ". We see that bohr’s theory of the hydrogen atom answers the question as to why this previously known formula describes the hydrogen spectrum. it is because the energy levels are proportional to 1 n2, where n is a non negative integer. a downward transition releases energy, and so ni must be greater than nf.
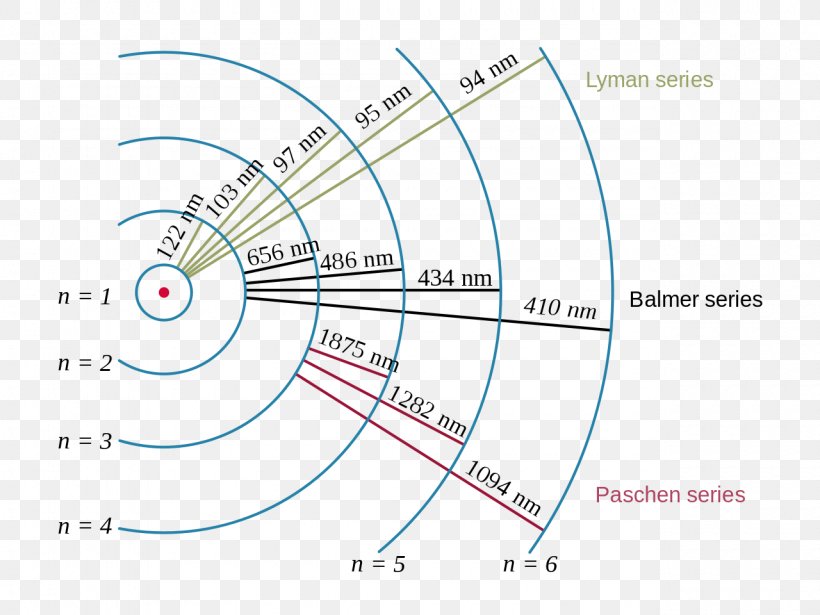
Energy Level Hydrogen Atom Bohr Model Hydrogen Spectral ођ In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. according to bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. each orbit has its specific energy level, which is expressed as a negative value. this is because the electrons on the orbit are ". We see that bohr’s theory of the hydrogen atom answers the question as to why this previously known formula describes the hydrogen spectrum. it is because the energy levels are proportional to 1 n2, where n is a non negative integer. a downward transition releases energy, and so ni must be greater than nf. In this state the radius of the orbit is also infinite. the atom has been ionized. figure 5.4.2: the bohr model of the hydrogen atom (a) the distance of the orbit from the nucleus increases with increasing n. (b) the energy of the orbit becomes increasingly less negative with increasing n. Bohr’s model of the hydrogen atom is based on three postulates: (1) an electron moves around the nucleus in a circular orbit, (2) an electron’s angular momentum in the orbit is quantized, and (3) the change in an electron’s energy as it makes a quantum jump from one orbit to another is always accompanied by the emission or absorption of a photon.
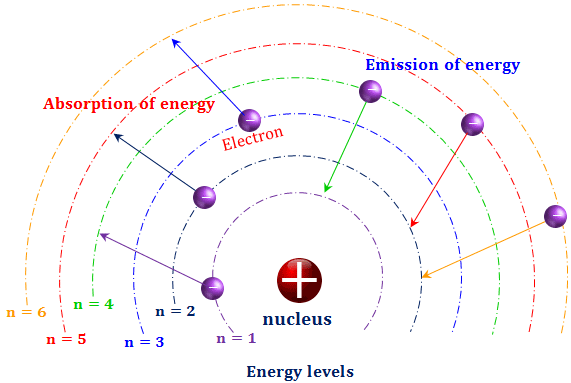
Energy Diagram For Hydrogen Atom In this state the radius of the orbit is also infinite. the atom has been ionized. figure 5.4.2: the bohr model of the hydrogen atom (a) the distance of the orbit from the nucleus increases with increasing n. (b) the energy of the orbit becomes increasingly less negative with increasing n. Bohr’s model of the hydrogen atom is based on three postulates: (1) an electron moves around the nucleus in a circular orbit, (2) an electron’s angular momentum in the orbit is quantized, and (3) the change in an electron’s energy as it makes a quantum jump from one orbit to another is always accompanied by the emission or absorption of a photon.

Comments are closed.