Alkali Metals Properties Electronic Configuration Periodic Trends
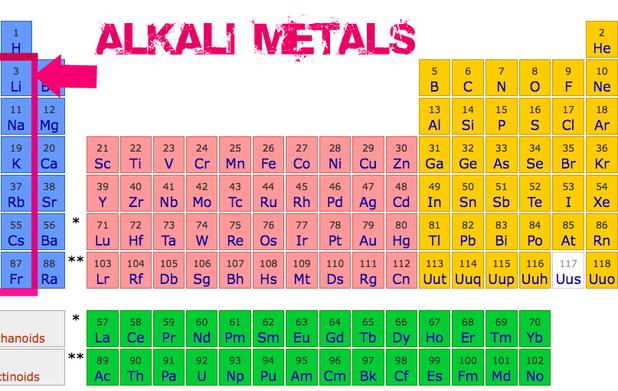
The General Properties Of The Alkali Metals In The Modern Periodic Alkali metals have a corresponding [noble gas] ns 1 electronic configuration. they occupy the first column of the periodic table. alkali elements are lithium (li), sodium (na), potassium (k), rubidium (ru), cesium (cs) and francium (fr), occupying successive periods from first to seven. francium is a radioactive element with a very low half life. Jim clark (chemguide.co.uk) group 1: properties of alkali metals is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by libretexts. this page discusses the trends in some atomic and physical properties of the group 1 elements lithium, sodium, potassium, rubidium and cesium.

Alkali Metal Definition Location In Periodic Table Properties Alkali metals properties, electronic configuration, and periodic trends. alkali metals are a group of chemical elements found in the first column of the periodic table. these metals are highly reactive and are known for their ability to easily lose their outermost electron, resulting in the formation of positively charged ions. alkali metals. General properties of the alkali metals. various properties of the group 1 elements are summarized in table 20.4.1 20.4. 1. in keeping with overall periodic trends, the atomic and ionic radii increase smoothly from li to cs, and the first ionization energies decrease as the atoms become larger. Electronic configuration of alkali metals. alkali metals are the metal that is placed in the first group of the periodic table. they have one electron in their outermost shell. the general electronic configuration of alkali metals is ns1. for example, the electronic configuration of lithium is 1s2, 2s1. The alkali metals, also known as the alkali metal family is a group of six elements characterized by common physical and chemical properties, a similar electron configuration, and shared periodic trends. all the alkali metals are found in nature, but being highly reactive, they do not occur freely in their pure form [1].

Comments are closed.