Alkali Metals Chemical Elements Properties Alkali Metals Periodic

Alkali Metal Definition Properties Facts Britannica The alkali metals have the silver like lustre, high ductility, and excellent conductivity of electricity and heat generally associated with metals. lithium is the lightest metallic element. the alkali metals have low melting points, ranging from a high of 179 °c (354 °f) for lithium to a low of 28.5 °c (83.3 °f) for cesium. Alkali metals are the six different chemical elements found in the first column of the periodic table: lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs) and francium (fr). the alkali metals group is part of the s block of elements in the periodic table, that along with hydrogen, helium, calcium and others, have their.
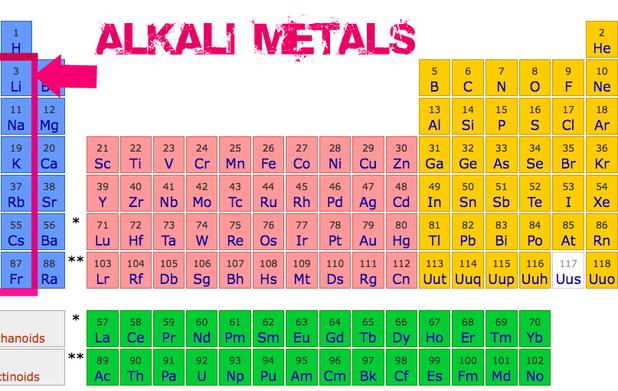
The General Properties Of The Alkali Metals In The Modern Periodic The lone outer shell electrons leads the alkali metal elements to share several common properties: the one outer electron is easily lost, forming the univalent (1 ) cation (e.g., na ). alkali metals are typically less dense than other metals. atoms of alkali metals have the largest atomic radii of elements in their periods. The alkali metals are among the most electropositive elements on the periodic table and thus tend to bond ionically to the most electronegative elements on the periodic table, the halogens (fluorine, chlorine, bromine, iodine, and astatine), forming salts known as the alkali metal halides. the reaction is very vigorous and can sometimes result. Alkali metal reactivity, group 1, properties: since the alkali metals are the most electropositive (the least electronegative) of elements, they react with a great variety of nonmetals. in its chemical reactivity, lithium more closely resembles group 2 (iia) of the periodic table than it does the other metals of its own group. it is less reactive than the other alkali metals with water. General properties of the alkali metals. various properties of the group 1 elements are summarized in table 20.4.1 20.4. 1. in keeping with overall periodic trends, the atomic and ionic radii increase smoothly from li to cs, and the first ionization energies decrease as the atoms become larger.
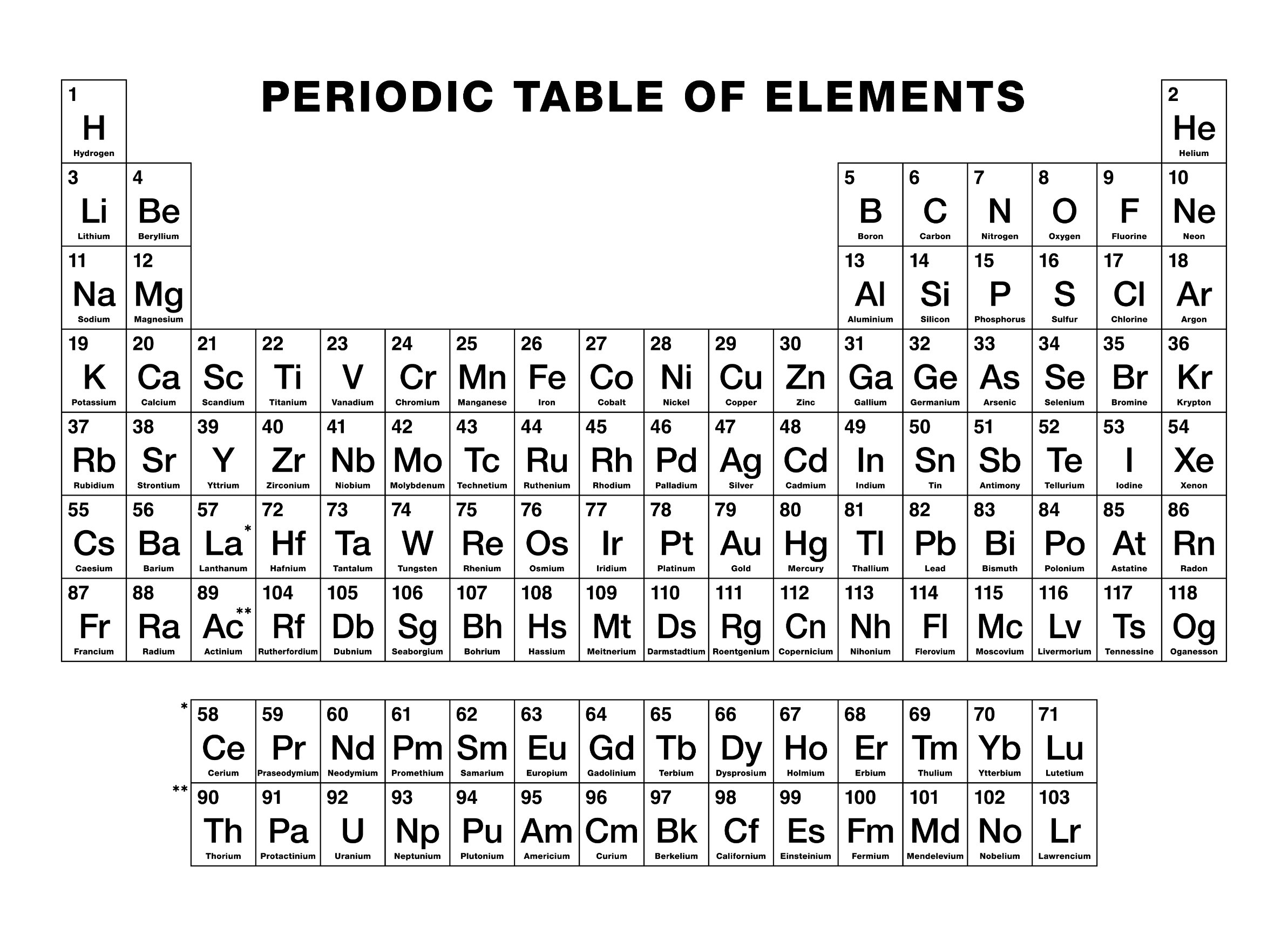
Periodic Table Of Elements Alkali Metals Alkali metal reactivity, group 1, properties: since the alkali metals are the most electropositive (the least electronegative) of elements, they react with a great variety of nonmetals. in its chemical reactivity, lithium more closely resembles group 2 (iia) of the periodic table than it does the other metals of its own group. it is less reactive than the other alkali metals with water. General properties of the alkali metals. various properties of the group 1 elements are summarized in table 20.4.1 20.4. 1. in keeping with overall periodic trends, the atomic and ionic radii increase smoothly from li to cs, and the first ionization energies decrease as the atoms become larger. Alkali metal properties. the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. alkali metals have one electron in their outer shell, which is loosely bound. this gives them the largest atomic radii of the elements in their respective periods. The alkali metals, also known as the alkali metal family is a group of six elements characterized by common physical and chemical properties, a similar electron configuration, and shared periodic trends. all the alkali metals are found in nature, but being highly reactive, they do not occur freely in their pure form [1].

Alkali Metals Chemistry Alkali metal properties. the alkali metals exhibit many of the physical properties common to metals, although their densities are lower than those of other metals. alkali metals have one electron in their outer shell, which is loosely bound. this gives them the largest atomic radii of the elements in their respective periods. The alkali metals, also known as the alkali metal family is a group of six elements characterized by common physical and chemical properties, a similar electron configuration, and shared periodic trends. all the alkali metals are found in nature, but being highly reactive, they do not occur freely in their pure form [1].
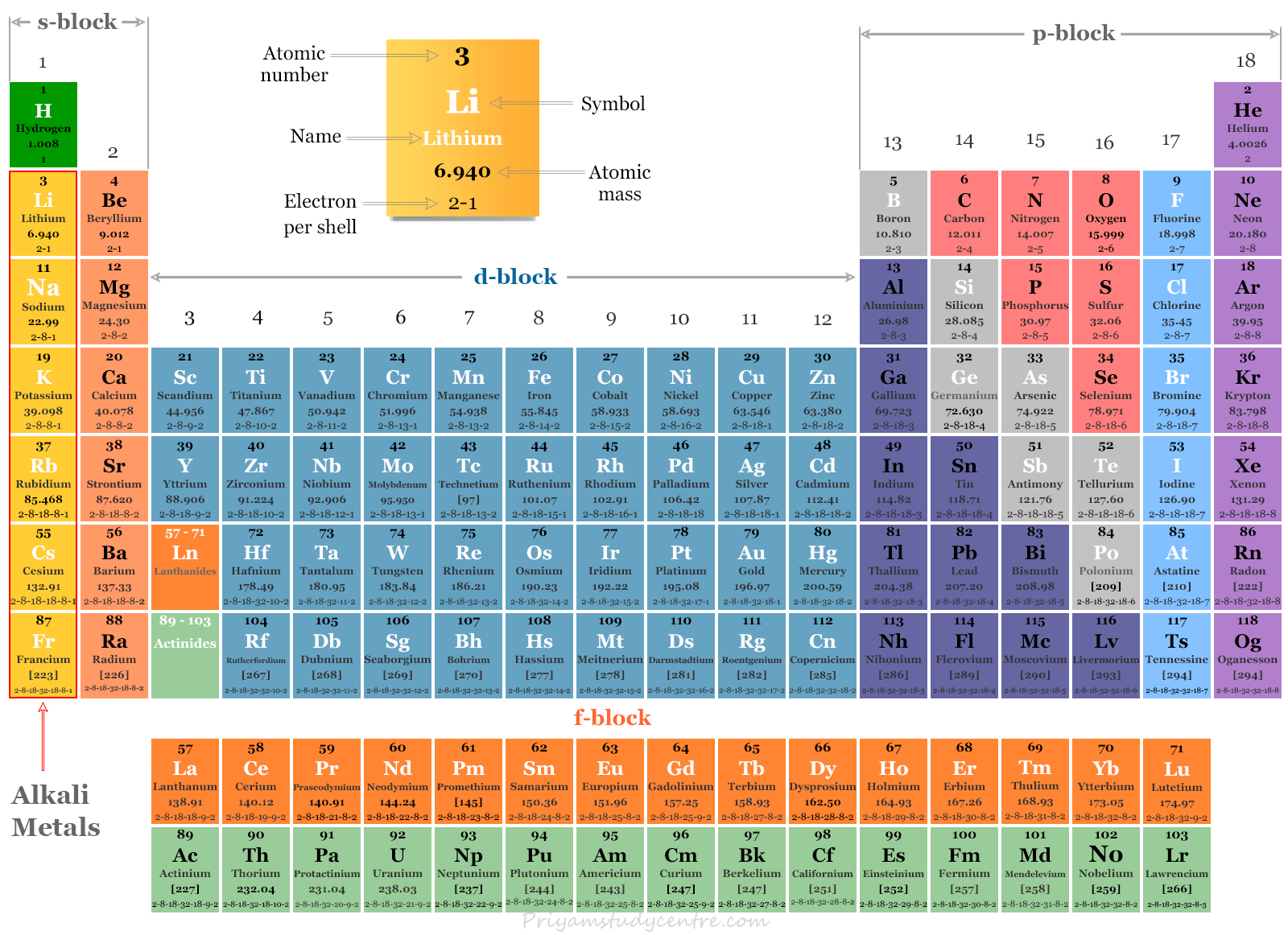
Periodic Table Of Elements Alkali Metals

Comments are closed.