A Reversible Reaction Of Hydrated Copper Ii Sulfate Experiment Rsc
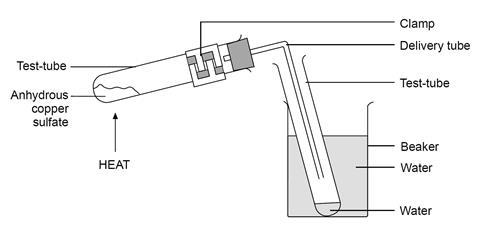
A Reversible Reaction Of Hydrated Copper Ii Sulfate Experiment Rsc The reaction involved is: cuso 4.5h 2 o (s) (pale blue solid) ⇌ cuso 4 (s) (“dirty” white solid) 5h 2 o (l) students should observe the colour change from pale blue to white and the change back to blue when water is added. the colour change on adding water to anhydrous copper (ii) sulfate has been used as a test for the presence of. The reversible copper sulfate reaction. when concentrated hydrochloric acid is added to a very dilute solution of copper sulfate, the pale blue solution slowly turns yellow green on the formation of a copper chloride complex. when concentrated ammonia solution is added, copious quantities of white smoke are produced, heat is generated and the.
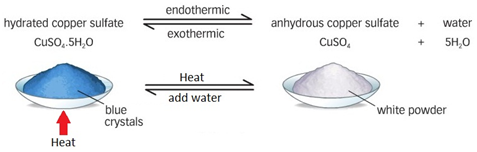
Copper 2 Sulfate With Water Heat A reversible reaction of hydrated copper(ii) sulfate. in association with nuffield foundation. a class practical which investigates the reversible reaction of hydrated copper(ii) sulfate. includes kit list and safety instructions. How to carry out the reversible reaction of hydrated copper sulphate. Blue copper(ii) sulfate is hydrated with five h₂o molecules for every pair of copper(ii) and sulfate ions. upon heating an endothermic change takes place as. Edexcel. spanish. past papers. cie. spanish language & literature. past papers. other subjects. revision notes on reversible reactions for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams.

Comments are closed.